NCERT QUESTIONS: CHAPTER 4: STRUCTURE OF THE ATOM
NCERT INTEXT QUESTIONS(P-39)
- What are canal rays?
Canal rays are positively charged radiations consisting of particles that have a charge equal in magnitude but opposite in sign to that of electrons. The mass of canal rays depends upon the nature of gas. It led to the discovery of a positively charged sub-atomic particle called a proton. - If an atom contains one electron and one proton, will it carry any charge or not?
Since the magnitude of charge on an electron and proton is the same, the atom will be electrically neutral as one negative charge balances one positive charge.
NCERT INTEXT QUESTIONS(P-41)
- On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
The negative and positive charges are equal in number and magnitude. So, the atom as a whole is electrically neutral. - On the basis of Rutherford’s model of an atom, which sub-atomic particle is present in the nucleus of an atom?
As per Rutherford’s model of an atom, the protons which are positively charged are present in the nucleus of an atom. - Draw a sketch of Bohr’s model of an atom with three shells.
- What do you think would be the observation if the α-particle scattering experiment is carried out using a foil of a metal other than gold?
If α-particle scattering experiment is carried out using a foil of any metal as thin as gold foil used by Rutherford, there would be no change in observation. But, since all metals are not equally malleable and such a thin foil is difficult to obtain and if you use a thick foil, then more α-particles would bounce back and no idea about the location of positive mass in the atom would be available with such a certainty.
NCERT INTEXT QUESTIONS (P-41 Contd.)
- Name the three sub-atomic particles of an atom.
The sub-atomic particles of an atom are
(a) Electron, e, (b) Proton, H and (c) neutron, n - Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Atomic mass = number of protons + number of neutrons
4 = 2 + number of neutrons
Number of neutrons = 4 – 2 = 2
Hence, Helium has 2 neutrons.
NCERT INTEXT QUESTIONS(P-42)
- Write the distribution of electrons in carbon and sodium atoms.
The total number of electrons in carbon atom is 6. So, the distribution of electrons in carbon atom is given by:
First orbit or K-shell = 2 electrons
Second orbit or L-shell = 4 electrons
So, we can write the distribution of electrons in a carbon atom as 2,4.
The total number of electrons in sodium atom is 11. So, the distribution of electrons in carbon atom is given by:
First orbit or K-shell = 2 electrons
Second orbit or L-shell = 8 electrons
Third orbit or K-shell = 1 electron
So, we can write the distribution of electrons in a sodium atom as 2,8,1.
- If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Maximum number of electrons in K-shell = 2
Maximum number of electrons in L-shell = 8
If K and L-shells of an atom are full,
then the total number of electrons in the atom would be (2 + 8) = 10 electrons.
NCERT INTEXT QUESTIONS(P-44)
- How will you find the valency of chlorine, sulphur and magnesium?
Valency of Chlorine:
The electronic configuration of chlorine = 2, 8, 7.
Since, chlorine has 7 (more than 4) electrons in its outermost shell.
- Valency of chlorine = 8 – number of electrons in outermost shell = 8−7 = 1
Valency of Sulphur:
The electronic configuration of Sulphur = 2, 8, 6.
Since, Sulphur has 6 (more than 4) electrons in its outermost shell.
- Valency of sulphur = 8-number of electrons in outermost shell = 8−6 = 2.
Valency of Magnesium:
The electronic configuration of Magnesium = 2, 8, 2.
Since, magnesium has 2 (less than 4) electrons in its outermost shell.
- Valency of magnesium= Number of electrons in its outermost shell = 2
NCERT INTEXT QUESTIONS (P-44 Contd.)
- If number of electrons in an atom is 8 and number of protons is also 8, then
(i) What is the atomic number of the atom?
(i) The atomic number of an atom is the same as the number of protons in that atom, hence its atomic number is 8.
(ii) What is the charge on the atom?
(ii) In an atom, the number of protons is equal to the number of electrons. Hence both the charges – positive and negative neutralize each other. Therefore, the atom does not possess any charge. www.physicsinduction.com
- With the help of given Table, find out the mass number of oxygen and sulphur atom.
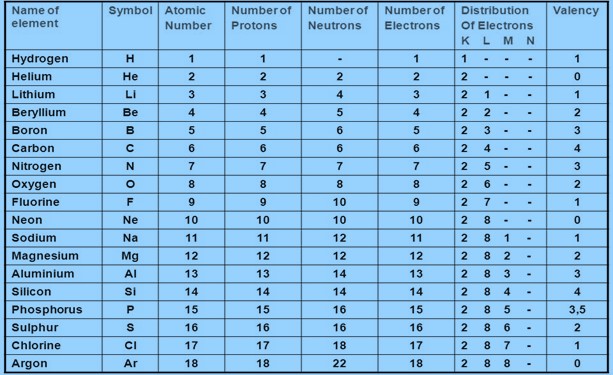
(a) To find the mass number of Oxygen:
Number of protons = 8
Number of neutrons = 8
Atomic number = 8
Atomic mass number = Number of protons + number of neutrons = 8 + 8 = 16
Therefore, mass number of oxygen = 16
(b) To find the mass number of Sulphur:
Number of protons = 16
Number of neutrons = 16
Atomic number = 16
Atomic mass number = Number of protons + number of neutrons = 16 + 16 = 32
Therefore, mass number of sulphur = 32
NCERT INTEXT QUESTIONS(P-45)
- For the symbol H, D and T tabulate three sub-atomic particles found in each of them.
| Sub-atomic particles | H | D | T |
| Electron | 1 | 1 | 1 |
| Proton | 1 | 1 | 1 |
| neutron | 0 | 1 | 2 |
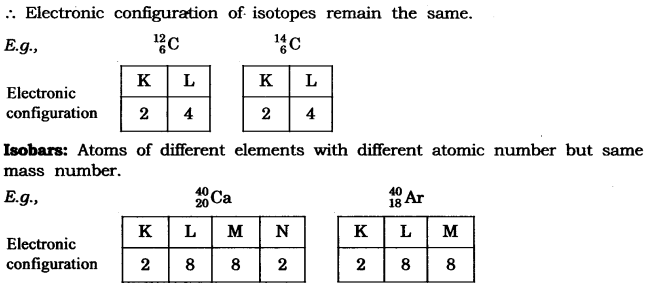
2. Write the electronic configuration of any one pair of isotopes and isobars.
NCERT EXERCISES (P-46 to 48)
- Compare the properties of electrons, protons and neutrons.
| Property | Electrons | Protons | Neutrons |
| Charge | Negatively charged | Positively charged | No charge. |
| Location | Located outside the nucleus | Located within the nucleus | Located inside the nucleus of an atom |
| Weight | Mass is negligible | 1 a.m.u | 1 a.m.u |
| Affinity | Attracted towards positively charged | Attracted towards negatively charged | Do not get attracted to any charged particle |
- What are the limitations of J.J. Thomson’s model of the atom?
According to J.J. Thomson’s model of an atom, the electrons are embedded all over in the positively charged spheres. But experiments done by other scientists showed that protons are present only in the centre of the atom and electrons are distributed around it. - What are the limitations of Rutherford’s model of the atom?
According to Rutherford’s model of an atom the electrons are revolving in a circular orbit around the nucleus. Any such particle that revolves would undergo acceleration and radiate energy. The revolving electron would lose its energy and finally fall into the nucleus, the atom would be highly unstable. But we know that atoms are quite stable. - Describe Bohr’s model of the atom.
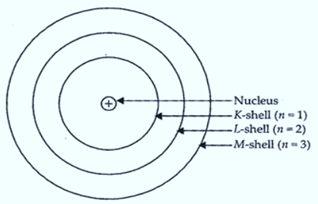
Bohr’s model of the atom
(a) Atom has nucleus in the centre.
(b) Electrons revolve around the nucleus in discrete orbits called shells or energy levels.
(c) These orbits or shells are represented by the letters K, L, M, N or the numbers n = 1, 2, 3, 4.
(d) While revolving in discrete orbits, the electrons do not radiate energy.
- Compare all the proposed models of an atom given in this chapter.
J.J. Thomson: Electrons are embedded in a sphere of positive charge.
Rutherford: All the positive charge is located in a very small space. Electrons revolve around the nucleus in the extra nuclear space.
Neils Bohr: Electrons move round the nucleus in specific orbits. There is a loss or gain in energy of electron only when it moves from one orbit to another.
- Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Following rules are followed to fill the electrons in different energy levels.
- If n gives the number of orbit or energy level, then 2n2 gives the maximum number of electrons possible in a given orbit or energy level. Thus
First orbit or K-shell will have 2n2= 2(1)2 = 2 electrons
Second orbit or L-shell will have 2n2= 2(2)2 = 8 electrons
Third orbit or M-shell will have 2n2= 2(3)2 = 18 electrons
- The maximum number of electrons that can be accommodated in the outermost orbit is 8.
- Electrons are not accommodated in a given shell unless the inner shells are filled. (Shells are filled step-wise).
Thus, 18 electrons will be accommodated as:
K=2, L=8, M=8
- Define valency by taking examples of silicon and oxygen.
Valency is the combining capacity of an atom.
Atomic number of oxygen = 8, Atomic number of silicon = 14
Electronic configuration of oxygen = 2, 6
Electronic configuration of silicon =2, 8, 4
In the atoms of oxygen, the valence electrons are 6 (i.e., electrons in the outermost shell). To fill the orbit, 2 electrons are required. In the atom of silicon, the valence electrons are 4. To fill this orbit 4 electrons are required.
Hence, the combining capacity of oxygen is 2 and of silicon is 4.
e., Valency of oxygen = 2
Valency of silicon = 4 - Explain with examples: (i) Atomic number, (ii) Mass number, (iii) Isotopes and (iv) Isobars. Give any two uses of isotopes.
(i) Atomic number: The atomic number of an element is equal to the number of protons in the nucleus of its atom.
for example: Carbon has 6 protons hence atomic no. = 6.
(ii) Mass number: The mass number of an atom is the sum of the total number of protons and neutrons present in the nucleus of an atom.
for example: there are 6 protons and 6 neutrons in the nucleus of an atom, so its mass number is 12.
(iii) Isotopes: Isotopes are atoms of the same element which have different mass number but same atomic number.
![]()
(iv) Isobars: Isobars are atoms having the same mass number but different atomic numbers.
![]()
Both calcium and argon have same mass number but different atomic number.
Two uses of isotopes are:
(i) An isotope of iodine is used in the treatment of goitre.
(ii) An isotope of uranium is used as a fuel in nuclear reactors.
- Na+has completely filled K and L shells. Explain.
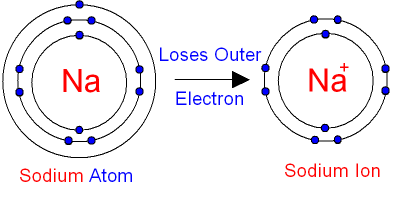
Atomic number of Na = 11
Number of electrons =11
The electronic configuration = 2, 8, 1.
Sodium atom (Na) looses 1 electron to become stable and form Na+ ion. Hence it has completely filled K and L shells.
The electronic configuration of Na+ ion = 2 (K-shell), 8 (L-shell)
Thereby Na+ ion has completely filled K and L shells.
10. If bromine atom is available in the form of say, two isotopes 7935Br (49.7%) and 8135Br (50.3%), calculate the average atomic mass of bromine atom.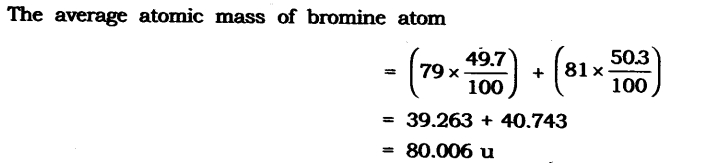
11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 168X and 188X in the sample?
Let the percentage of 168X be x and the percentage of 168X be 100 – x.
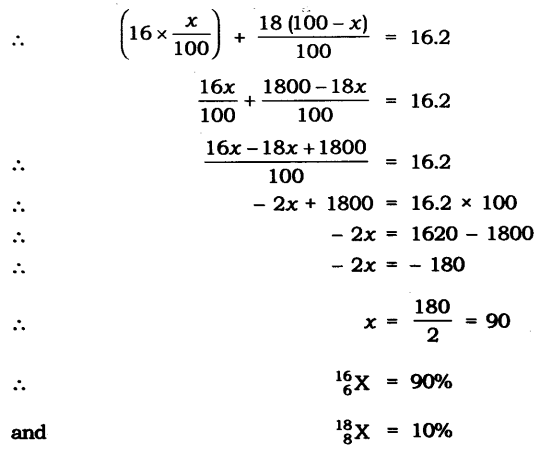
12. If Z = 3, what would be the valency of the element? Also, name the element.
Z = 3, (i.e, atomic number —> z)
∴ Electronic configuration = 2, 1
Valency = 1
The name of the element is lithium.
13. The composition of the nuclei of two atomic species X and Y are given as under
| X | Y | |
| Protons = | 6 | 6 |
| Neutrons = | 6 | 8 |
Give the mass numbers of X and Y. What is the relation between the two species?
Mass number = Number of protons + Number of neutrons
Mass number of X = 6 + 6 = 12
Mass number of Y = 6 + 8 = 14
Atomic number = Number of protons
These two atomic species have the same atomic number but different mass numbers,
Hence, both X and Y are isotopes of same element.
14. For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
(a) False (b) False (c) True (d) False
Put tick against correct choice and cross (x) against wrong choice in questions 15, 16 and 17.
15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus (b)Electron (c) Proton (d)neutron
(a) Atomic nucleus
16. Isotopes of an element have
(a) the same physical properties (c) different number of neutrons
(b)different number of neutrons (d) different atomic numbers.
(c) different number of neutrons
17. The number of valence electrons in Cl– ion are :
(a) 16 (b) 8 (c) 17 (d) 18
(b) 8
18. Which one of the following is a correct electronic configuration of sodium?
(a) 2, 8 (b) 8, 2, 1 (c) 2, 1, 8 (d) 2, 8, 1
(d) 2, 8, 1
19. Complete the following table.
| Atomic number | Mass number | Number of neutrons | Number of Protons | Number of electrons | Name of the atomic species |
|
9 16 – – – |
–
32 24 2 1 |
10
– – – 0 |
–
– 12 1 1 |
–
– – – 0 |
– Sulphur – – – |
| Atomic number | Mass number | Number of neutrons | Number of Protons | Number of electrons | Name of the atomic species |
|
9 16 12 1 1 |
19
32 24 2 1 |
10
16 12 1 0 |
9
16 12 1 1 |
9
6 12 1 0 |
Fluorine Sulphur Magnesium Deuterium Hydrogen |
